Corrosion: How Oxidation Degrades Your Metal Product and Your Profit!
Surface corrosion is certainly a headache for any metal finishing operation. It is very important for finishers to prevent and treat corrosion properly for consistent and positive finishing results. Giering Metal Finishing presents the following input to address this global issue:
What Is Corrosion?
Corrosion is the oxidation of metals by water, oxygen, chemicals and other metals. Oxidation is also referred to as “rust” mostly for ferritic metals and also “white rust” for aluminum, zinc and other alloys.
There are basically 4 types of corrosion:
- Surface corrosion, which is the initial stage, appearing as a light, crust-like formation on the top layer of the metal.
- Filiform corrosion undermines a surface finish, looking like little worms or filaments spreading under the coating into metal. It can only occur when there is a coating or layer over the top of metal.
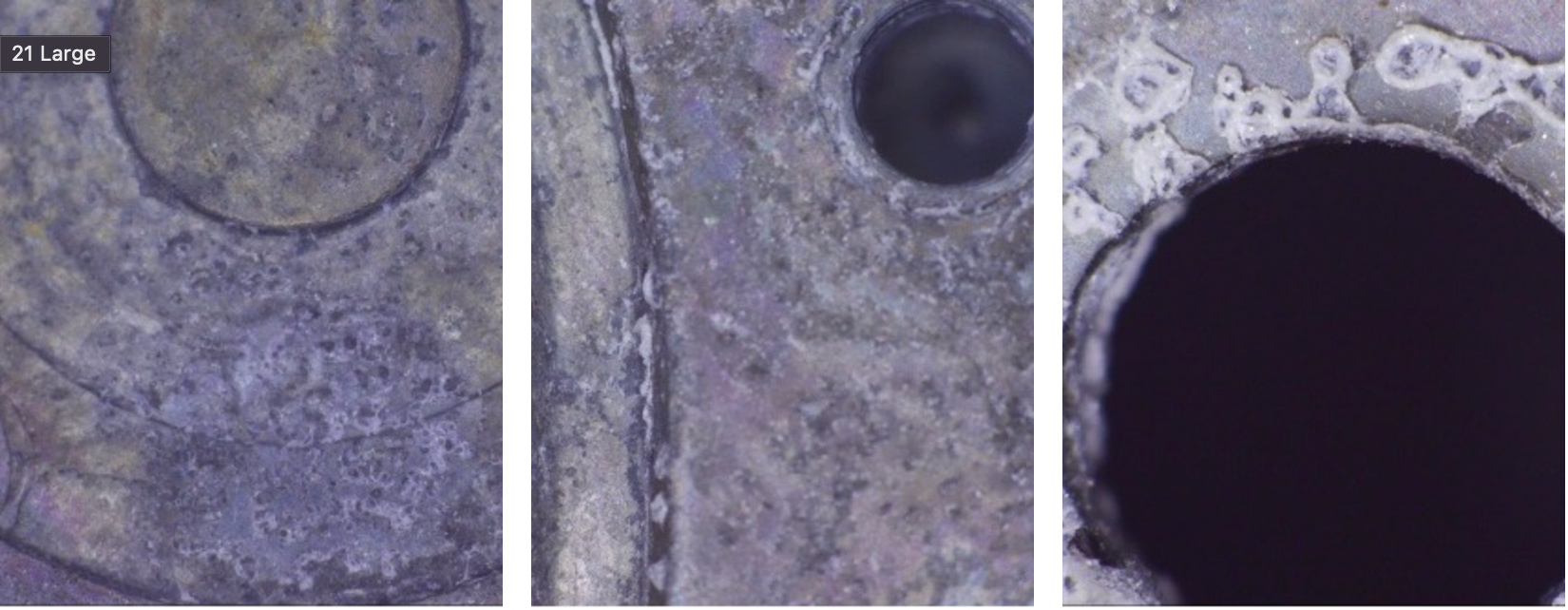
- Pitting corrosion is a severe form, beyond surface oxidation which results in holes and cavities within the material.
- Crevice corrosion which occurs in the presence of water and often occurs at joints such as bolted connections.
Why Is Corrosion a Problem?
When materials corrode, the metal can:
- lose structural strength and create safety issues.
- undermine the adhesion of any topcoat.
- change appearance and become unsightly.
- cause contamination and health issues
- make parts very expensive to repair.
- make parts less efficient and less valuable (particularly with electrical components)
How Does Giering Metal Finishing Address Corrosion?
We inspect all incoming product to make sure that there is no oxidation (rust) on bare parts. If there is oxidation, the corroded metal surface can be restored mechanically or sometimes chemically in the following processes:
- Acid etch, commonly referred to as acid pickle, eats away at the oxides. These are immersion baths typically integrated within a series of baths for a cleaning, pretreatment, conversion coating or some form of plating line. Acids are also used to remove flux/slag from welding, laser cutting and other fabrication processes that leave oxides on the metal surface. Unfortunately, chemicals work best for only surface rust and medium grade pitting. If rust is severe, abrasion is the best method to remove the oxides down to the core.
- Blasting with the appropriate media to achieve the level of abrasion and surface finish desired (aluminum oxide, glass bead, plastic bead, walnut shells, baking soda, etc)
- Several types of abrasion techniques including sanding (various grit types), grinding, wire brush or wheel, delivered either manually or by power-driven tools depending on the application.
- Once the surface is corrosion free, we can apply the pretreatment specified, like a conversion coating (iron phosphate, zinc phosphate, chromate or zirconium), to condition and protect the parts prior to top coating.
- For additional protection, we can apply an epoxy electrocoating to the metal ( aka ecoat or electrodeposition coating). This coating is applied thru an electrically charged aqueous bath.
- Ecoat is a very robust coating, serving as an excellent primer or final coat.
- Ecoat is the primer coating that car manufacturers use to protect our cars from rusting.
- Then we can apply an additional topcoat of powder coat or wet paint in any color that you choose to seal up the surface.
- We know that these 3 steps work in most applications because this multi-step process passes an accelerated 1000+ hour salt-spray corrosion test per ASTM B1117. This test is a very high standard for outdoor corrosion resistance.
What Steps Can I Take to Minimize the Chances of Corrosion?
- Store your parts in a dry environment.
- Try to control the relative humidity in your storage area.
- Prevent transferring parts from a controlled environment to uncontrolled or vice versa, especially in the warm and humid summer months. This can introduce condensation which commonly causes corrosion. Even transporting parts from cool controlled environments to a hot humid truck can cause problems.
- Late June through early September is known as “the season to be rusty”. Hot temperatures, high dew points and high humidity cause hindering conditions for raw metal.
- If parts are transferred, properly protect parts in a VCI (Vapor Corrosion Inhibitor) bag or desiccant to prevent condensation.
- Protect parts that will eventually be coated with a corrosion inhibiting oil/rust preventative, a barrier film that prevents oxidation. Rust preventative should be light, water based (not oil based), and easy to remove with aqueous cleaners. Make sure that it’s not too heavy and does not dry onto the surface. Also, never use grease as it is very hard to remove with conventional cleaners and typically requires hot solvent vapor degreasing (trichloroethylene).
- If you’re purchasing parts from overseas that are being shipped via ocean freight, keep the materials in the shipping containers until they adjust to ambient temperatures. This is a very important issue. If you receive material via an ocean carrier and immediately open the boxes in a warm facility while the parts are still cold, you will introduce condensation. The material will begin to “sweat”, causing oxidation before we have a chance to coat the parts. This will undermine adhesion and the chemical durability of your product.

“Surface rust” forming on steel surface which is becoming “pitted rust”.
Oxidation forming on a zinc die cast which is typically referred to as “white rust”.